Eu Clinical Trial Regulation
The European Union (EU) Clinical Trial Regulation is a comprehensive set of regulations that govern the conduct of clinical trials across member states of the European Union. These regulations aim to streamline the process of conducting clinical trials, ensuring patient safety, and harmonizing the requirements for conducting trials within the EU. In this article, we will explore key aspects of the EU Clinical Trial Regulation and its impact on the pharmaceutical industry.
Regulatory Information
The EU Clinical Trial Regulation brings significant changes to the regulatory landscape, providing a unified framework for the authorization and supervision of clinical trials across all EU member states. The regulation applies to all clinical trials conducted within the European Union and covers both commercial and non-commercial trials.
Under the EU Clinical Trial Regulation, the responsibility for authorizing and overseeing clinical trials has shifted from individual member states to the European Medicines Agency (EMA). This centralization aims to simplify the approval process and harmonize the requirements for conducting clinical trials within the EU.
The regulation introduces a new EU Clinical Trials Database, which will serve as a central repository for all information related to authorized clinical trials. This database will improve transparency and facilitate access to information on ongoing and completed trials, ensuring greater accountability and accessibility of clinical trial data.
GMP for IMPs in View of the Revision of the EU Clinical Trial Provisions

Good Manufacturing Practice (GMP) guidelines for Investigational Medicinal Products (IMPs) play a crucial role in ensuring the quality and safety of products used in clinical trials. With the revision of the EU Clinical Trial Provisions, there are updates to the GMP requirements for IMPs.
Under the revised regulations, manufacturers of IMPs must adhere to the principles of GMP throughout the entire lifecycle of the product. This includes the sourcing of raw materials, manufacturing, packaging, labeling, storage, and distribution. The aim is to ensure that IMPs used in clinical trials meet the highest standards of quality and are produced in a consistent and reliable manner.
Furthermore, the EU Clinical Trial Regulation emphasizes the importance of risk management in the manufacturing of IMPs. Manufacturers must implement robust quality control systems to identify, assess, and mitigate risks associated with the production of IMPs. This includes conducting regular audits, inspections, and thorough documentation of all manufacturing processes.
Improved Patient Safety Measures
One of the primary goals of the EU Clinical Trial Regulation is to enhance patient safety during the conduct of clinical trials. The regulation introduces several measures to achieve this objective.
Firstly, the regulation requires the appointment of a qualified person responsible for pharmacovigilance (QPPV) for each clinical trial. The QPPV is responsible for ensuring the safety of trial participants and monitoring adverse events associated with the investigational product. This role is essential in promptly identifying and addressing any safety concerns that may arise during the trial.
Secondly, the EU Clinical Trial Regulation mandates the use of electronic systems for the collection, verification, and reporting of trial data. Electronic systems enable real-time monitoring of trial participants, facilitating the early detection of safety issues and efficient reporting of adverse events.
Additionally, the regulation places greater emphasis on informed consent procedures. Trial sponsors must ensure that potential participants receive comprehensive and understandable information about the trial, its risks, benefits, and alternatives, allowing them to make informed decisions regarding their participation.
Listicle: Key Benefits of the EU Clinical Trial Regulation
The EU Clinical Trial Regulation brings several benefits to stakeholders involved in the conduct and oversight of clinical trials. Here are three key advantages:
-
Streamlined Approval Process

The centralized authorization process under the EU Clinical Trial Regulation eliminates the need to submit separate applications to multiple member states, reducing administrative burden and accelerating the approval timeline for clinical trials.
-
Enhanced Data Transparency

The EU Clinical Trials Database promotes transparency by making trial-related information readily accessible to the public. This transparency fosters trust and allows researchers to access existing trial data, potentially saving time and resources.
-
Harmonization of Requirements

The EU Clinical Trial Regulation harmonizes the requirements for conducting clinical trials across all member states. This harmonization simplifies the regulatory landscape, enhances patient safety, and facilitates multinational trials.
FAQ (Frequently Asked Questions)
Q: How will the EU Clinical Trial Regulation impact the timeline for conducting clinical trials?
A: The centralized authorization process and harmonized requirements are expected to expedite the approval timeline for clinical trials. However, the actual impact on the timeline may vary depending on factors such as the complexity of the trial and the availability of resources.
Q: Are non-commercial trials also subject to the EU Clinical Trial Regulation?
A: Yes, the EU Clinical Trial Regulation applies to both commercial and non-commercial trials conducted within the European Union.
Q: Will the EU Clinical Trial Regulation affect the reporting of adverse events during clinical trials?
A: Yes, the regulation mandates the use of electronic systems for the collection, verification, and reporting of trial data, including adverse events. This facilitates real-time monitoring and efficient reporting of adverse events, enhancing patient safety.
In conclusion, the EU Clinical Trial Regulation represents a significant step forward in harmonizing and streamlining the regulatory framework for clinical trials in the European Union. The centralized authorization process, improved patient safety measures, and other key benefits contribute towards facilitating the conduct of safe and efficient clinical trials within the EU.
EU Clinical Trial Regulation - S-cubed Global
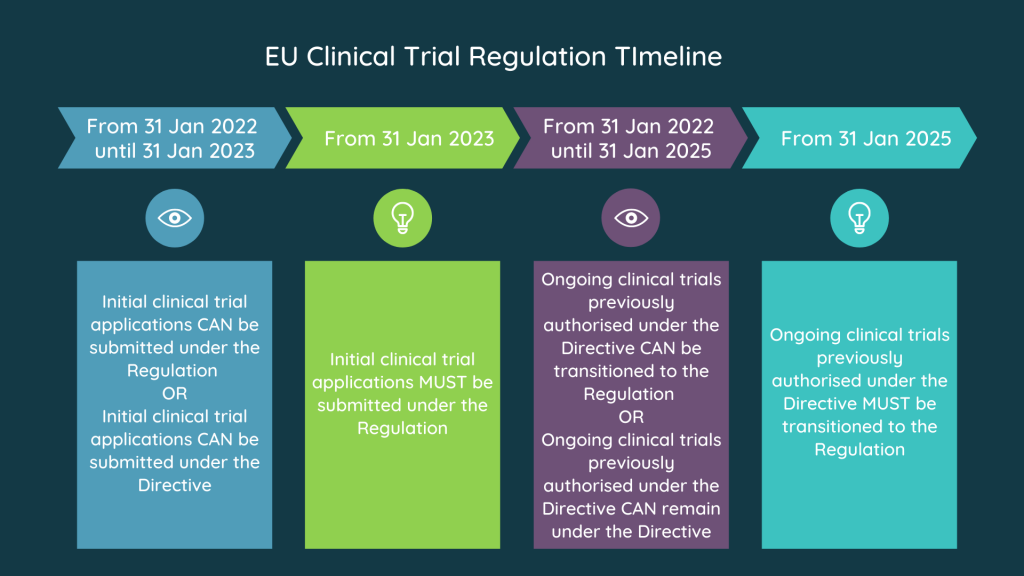 Image Source : www.s-cubed-global.com
Image Source : www.s-cubed-global.com EU Clinical Trials Regulation.. Summarised
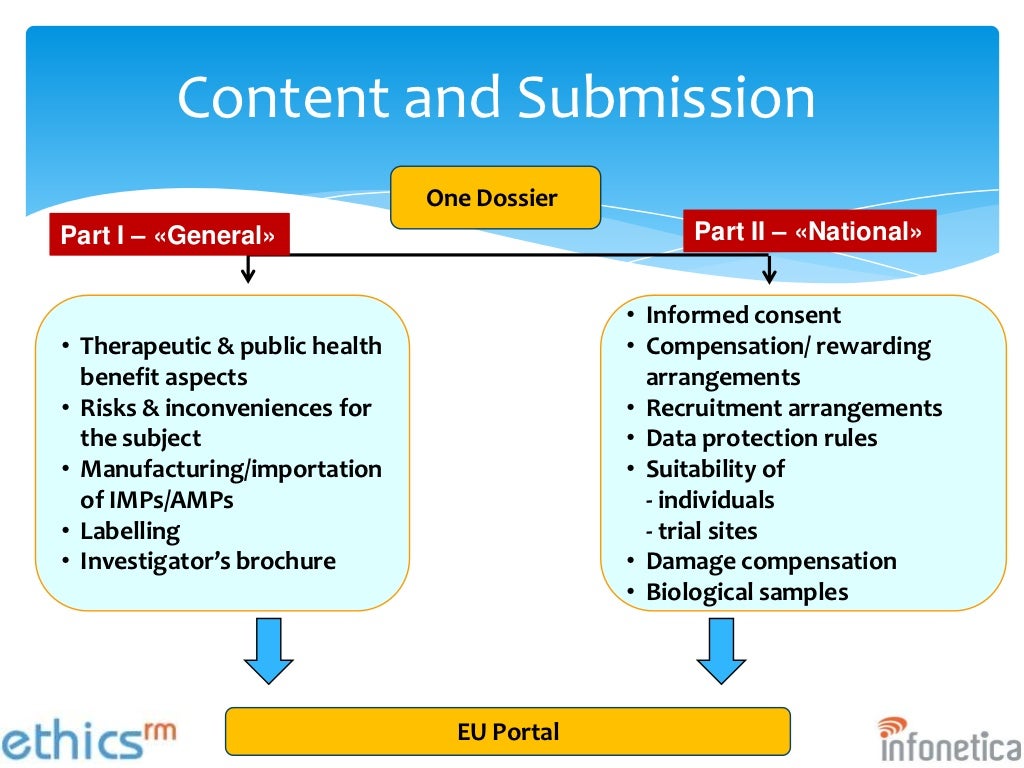 Image Source : www.slideshare.net
Image Source : www.slideshare.net trials summarised
EU Clinical Trial Regulation: Get Ready To Adapt!
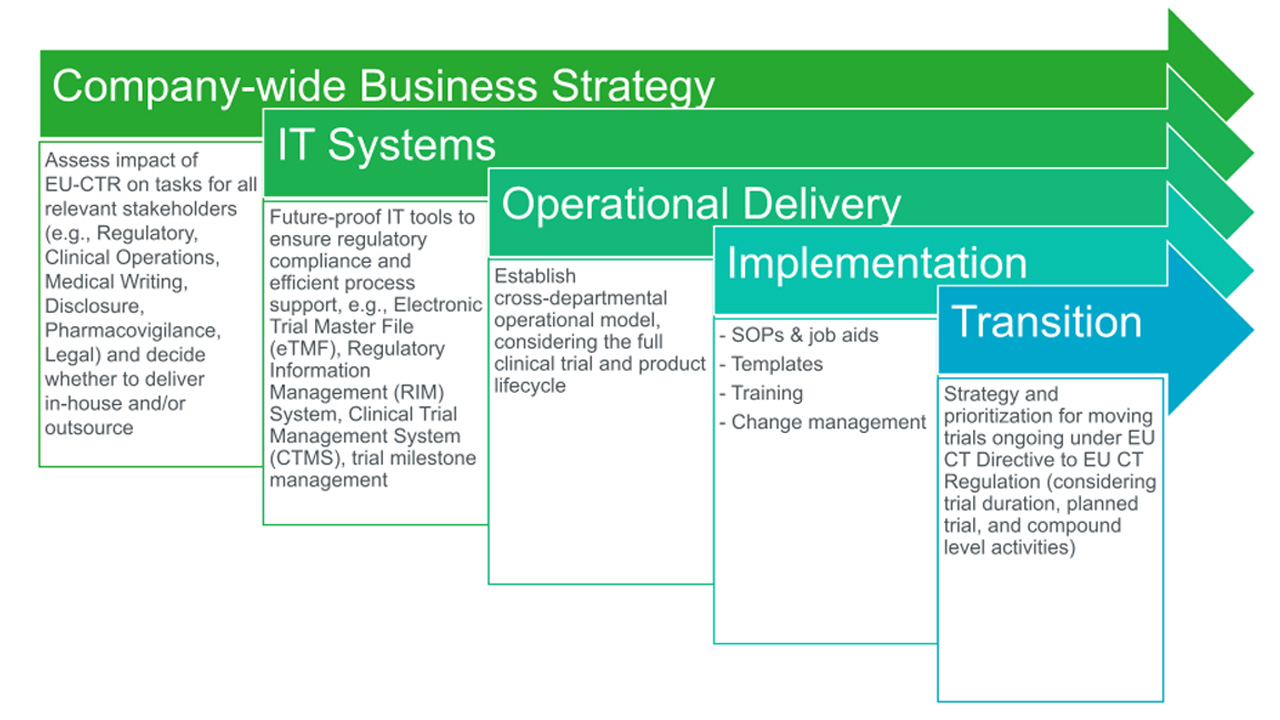 Image Source : globalforum.diaglobal.org
Image Source : globalforum.diaglobal.org EU Clinical Trial Regulation: Get Ready To Adapt!
 Image Source : globalforum.diaglobal.org
Image Source : globalforum.diaglobal.org New EU Clinical Trial Regulation And What It Means For Sponsors - Life
 Image Source : www.lslaw.co.uk
Image Source : www.lslaw.co.uk Regulatory Information
regulatory clinical trial regulation research information
GMP For IMPs In View Of The Revision Of The EU Clinical Trial Provisions
 Image Source : phact.ch
Image Source : phact.ch gmp imps provisions regulations trials
The EU Clinical Trial Regulation MasterClass
 Image Source : glceurope.com
Image Source : glceurope.com regulation masterclass
Regulation masterclass. Regulatory clinical trial regulation research information. Gmp imps provisions regulations trials. Eu clinical trial regulation. New eu clinical trial regulation and what it means for sponsors