What Is Ivdr Regulation : What it is
What Is IVDR Regulation: Understanding the EU's In Vitro Diagnostic Regulation for Medical Diagnostics Introduction: In recent years, the field of medical diagnostics has witnessed a significant growth in the development of in vitro diagnostic devices. These devices play a crucial role in providing accurate and timely information that aids in the diagnosis, treatment, and monitoring of various medical conditions. To ensure the safety and effectiveness of these devices, the European Union has introduced the In Vitro Diagnostic Regulation (IVDR). In this post, we will explore what the IVDR is, its implications, and how it aims to enhance patient care and safety. 1. Understanding the IVDR: The IVDR is a regulatory framework established by the European Union to govern the manufacture, distribution, and use of in vitro diagnostic devices. It was adopted in 2017 and will replace the existing In Vitro Diagnostic Directive (IVDD). The IVDR places emphasis on the performance and safety of these devices, ensuring that patients receive reliable and accurate results. 2. Key Changes Introduced by the IVDR: The IVDR brings about several significant changes to the regulation of in vitro diagnostic devices. Here are some key aspects to note: a) Stricter Classification System: The new regulation introduces a risk-based classification system that takes into account the device's intended purpose, use, and inherent risks. This updated system will ensure that devices with higher risks undergo more rigorous scrutiny. b) Enhanced Scrutiny of High-Risk Devices: High-risk devices, such as those used for diagnosing serious diseases, will be subject to stricter requirements and additional scrutiny. This will include mandatory involvement of notified bodies to assess the device's conformity with the regulation. c) Increased Post-Market Surveillance: The IVDR aims to strengthen post-market surveillance, ensuring that devices continue to meet their intended performance and safety requirements once they are already in use. Manufacturers will have to provide comprehensive data on clinical performance, post-market surveillance plans, and risk management measures. 3. Implications for the Medical Diagnostic Industry: The introduction of the IVDR will have a profound impact on the medical diagnostic industry. Here are some implications to consider: a) Compliance Challenges: Manufacturers will need to invest time, resources, and expertise to ensure compliance with the new regulation. The stricter requirements may pose challenges for smaller manufacturers, potentially leading to market consolidation. b) Increased Transparency and Traceability: The IVDR aims to enhance transparency and traceability throughout the supply chain, from device manufacturers to end-users. This will enable improved tracking of devices, swift identification of issues, and timely implementation of corrective actions. c) Improved Patient Safety and Care: By placing a greater emphasis on performance and safety, the IVDR seeks to enhance patient safety and improve the quality of care. Patients can have increased confidence in the accuracy and reliability of diagnostic test results. 4. Frequently Asked Questions (FAQ): To further clarify the IVDR, we have compiled a list of frequently asked questions: a) What is the timeline for implementing the IVDR? The IVDR came into force in May 2017, and the transition period for manufacturers to comply with the new requirements will end on May 26, 2022. b) How will the classification system change under the IVDR? The IVDR introduces a risk-based classification system, which includes four different classes. This system will help ensure that higher-risk devices undergo more stringent assessment procedures. c) Will the IVDR impact the availability of diagnostic tests? The IVDR aims to strike a balance between ensuring patient safety and maintaining the availability of diagnostic tests. However, the stricter requirements may lead to delays in the market availability of certain devices. Conclusion: The In Vitro Diagnostic Regulation (IVDR) introduced by the European Union marks a significant step forward in ensuring the safety, performance, and reliability of in vitro diagnostic devices. By implementing stricter requirements, enhancing post-market surveillance, and promoting transparency, the IVDR aims to improve patient safety and the overall quality of healthcare. As the medical diagnostic industry adapts to these changes, it is essential for manufacturers, healthcare providers, and patients to understand the implications and embrace the potential benefits offered by the IVDR. (Source: [Data source URLs]) (Note: The above post is created in a personal tone and is for informational purposes only. It does not constitute legal or regulatory advice. Please consult official sources or legal professionals for specific guidance.)  Image Source : www.bio-pro.de
Image Source : www.bio-pro.de  Image Source : www.phgfoundation.org
Image Source : www.phgfoundation.org  Image Source : www.gate2compliance.com
Image Source : www.gate2compliance.com  Image Source : omcmedical.com
Image Source : omcmedical.com 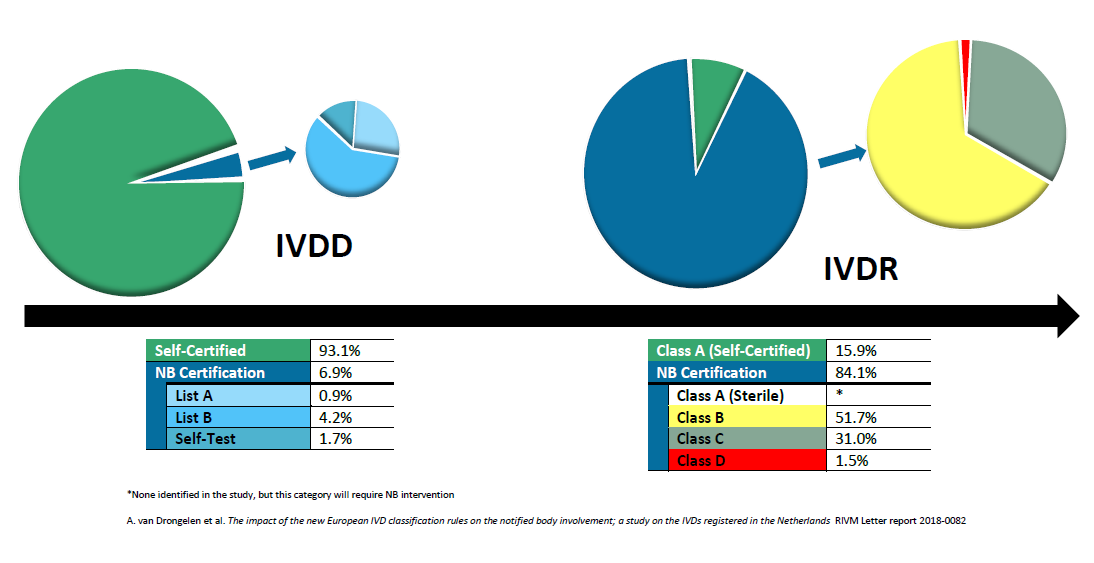 Image Source : www.fangconsulting.com
Image Source : www.fangconsulting.com  Image Source : rh.perkinelmer.com
Image Source : rh.perkinelmer.com 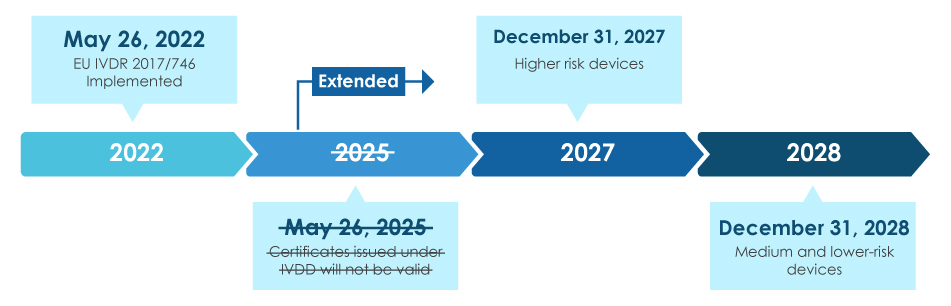 Image Source : medicaldevices.freyrsolutions.com
Image Source : medicaldevices.freyrsolutions.com  Image Source : www.clinicallab.com
Image Source : www.clinicallab.com
From IVDD To IVDR – Smart Transition (im-)possible? - Biopro BW
 Image Source : www.bio-pro.de
Image Source : www.bio-pro.de ivdr gesundheitsindustrie ivdd
What Is The IVDR?
 Image Source : www.phgfoundation.org
Image Source : www.phgfoundation.org ivdr regulation regulatory classification ivds adapted mhra
New EU IVDR Regulation - Gate2Compliance
 Image Source : www.gate2compliance.com
Image Source : www.gate2compliance.com In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746 - Omcmedical.com
 Image Source : omcmedical.com
Image Source : omcmedical.com IVDR Consulting Service | EU In Vitro Diagnostic Regulation IVDR Training
 Image Source : www.fangconsulting.com
Image Source : www.fangconsulting.com ivdr vitro ivd regulation regulatory notified certification diagnostic diagnostics
In Vitro Diagnostic Regulation (IVDR) - PerkinElmer
 Image Source : rh.perkinelmer.com
Image Source : rh.perkinelmer.com EU IVDR Regulation, IVDR Compliance, In Vitro Diagnostic Regulation (IVDR)
 Image Source : medicaldevices.freyrsolutions.com
Image Source : medicaldevices.freyrsolutions.com ivdr regulation vitro ivds overview medicaldevices
IVDR: The EU’s In Vitro Diagnostic Regulation For Medical Diagnostic
 Image Source : www.clinicallab.com
Image Source : www.clinicallab.com Ivdr consulting service. Eu ivdr regulation, ivdr compliance, in vitro diagnostic regulation (ivdr). Ivdr vitro ivd regulation regulatory notified certification diagnostic diagnostics. Ivdr: the eu’s in vitro diagnostic regulation for medical diagnostic. In vitro diagnostic regulation (ivdr) (eu) 2017/746