Medical Device Development Regulation And Law
Medical Device Development: Regulation and Law
Medical device development is a complex process that involves several stages, from ideation to commercialization. Throughout this journey, medical devices are subject to strict regulations and laws to ensure their safety and effectiveness. In this article, we delve into the world of medical device development regulation and law, shedding light on key aspects and providing valuable insights. So, let's dive right in!
1. Understanding Medical Device Regulations

Medical Device Development Book
One essential resource for gaining knowledge about medical device development regulation and law is the book titled "Sell, Buy or Rent Medical Device Development: Regulation and Law." Written by experts in the field, this book provides comprehensive information on the regulatory landscape surrounding medical devices.
2. Navigating the Regulatory Pathway

Amazon.com: Medical Device Development: Regulation and Law
Another valuable resource for immersing yourself in the realm of medical device development regulation and law is the book "Medical Device Development: Regulation and Law" available on Amazon. Authored by industry professionals, this book provides practical insights and guidelines on navigating the regulatory pathway.
3. Key Considerations in Medical Device Development
Developing a medical device that meets both regulatory requirements and customer needs requires careful attention to various factors. Here are three essential considerations in medical device development:
- Design Control: Ensuring that the device is designed in compliance with established regulations and standards is crucial. Design control processes, such as risk management, usability engineering, and verification and validation, are integral to this aspect.
- Clinical Evaluation: Before a medical device can be marketed, sufficient clinical data supporting its safety and performance must be gathered. Conducting clinical evaluations and trials in line with regulatory guidelines is essential to navigate this stage smoothly.
- Post-Market Surveillance: Monitoring the performance of medical devices after they enter the market is critical to ensure their continued safety and effectiveness. Implementing robust post-market surveillance processes helps identify and address any potential issues that may arise.
Frequently Asked Questions (FAQ) about Medical Device Development Regulation and Law
Q1: What is the role of regulatory bodies in medical device development?
A1: Regulatory bodies, such as the Food and Drug Administration (FDA) in the United States, play a vital role in overseeing medical device development. They establish and enforce regulations that aim to ensure the safety, effectiveness, and quality of medical devices that enter the market.
Q2: Are all medical devices subject to the same level of regulation?
A2: No, the level of regulation varies depending on the perceived risk of the medical device. Higher-risk devices, such as implantable devices, undergo more stringent regulatory scrutiny compared to low-risk devices like tongue depressors.
Q3: What are the consequences of non-compliance with medical device regulations?
A3: Non-compliance with medical device regulations can have serious consequences. Regulatory bodies have the authority to issue warnings, conduct inspections, initiate product recalls, and even impose fines or legal action against manufacturers who fail to comply with the established regulations.
Q4: Can medical device regulations differ between countries?
A4: Yes, medical device regulations can vary between countries. While some regulations are harmonized to align with global standards, there may be differences in specific requirements, submission processes, and approval timelines between different countries and regions.
Q5: How can medical device developers stay updated with changing regulations?
A5: Staying abreast of changing regulations is crucial for medical device developers. Subscribing to regulatory newsletters, attending conferences and webinars, participating in industry associations, and engaging with regulatory consultants can help developers stay informed and ensure compliance.
The Importance of Compliance
Ensuring compliance with medical device development regulation and law is of paramount importance. Compliance not only instills confidence in the device's safety and effectiveness but also helps companies avoid legal troubles and reputational harm. By adhering to regulations, manufacturers contribute to the overall improvement of the medical device ecosystem, benefiting both healthcare providers and patients.
Whether you are a professional in the medical device industry or an aspiring entrepreneur looking to embark on your own medical device development journey, understanding and navigating the world of medical device development regulation and law is indispensable. Equip yourself with knowledge, stay vigilant, and create innovative medical devices that have the potential to transform healthcare and save lives!
Disclaimer: The content presented in this article is for informational purposes only and should not be considered legal advice.
Medical Device Development: Regulation And Law 2020
 Image Source : www.hoganlovells.com
Image Source : www.hoganlovells.com Sell, Buy Or Rent Medical Device Development: Regulation And Law
 Image Source : booksrun.com
Image Source : booksrun.com Medical Device Development : Regulation And Law By Jonathan S. Kahan
 Image Source : www.ebay.com
Image Source : www.ebay.com Amazon.com: Medical Device Development: Regulation And Law
 Image Source : www.amazon.com
Image Source : www.amazon.com The 5 Medical Device Development Phases | Scilife (2022)
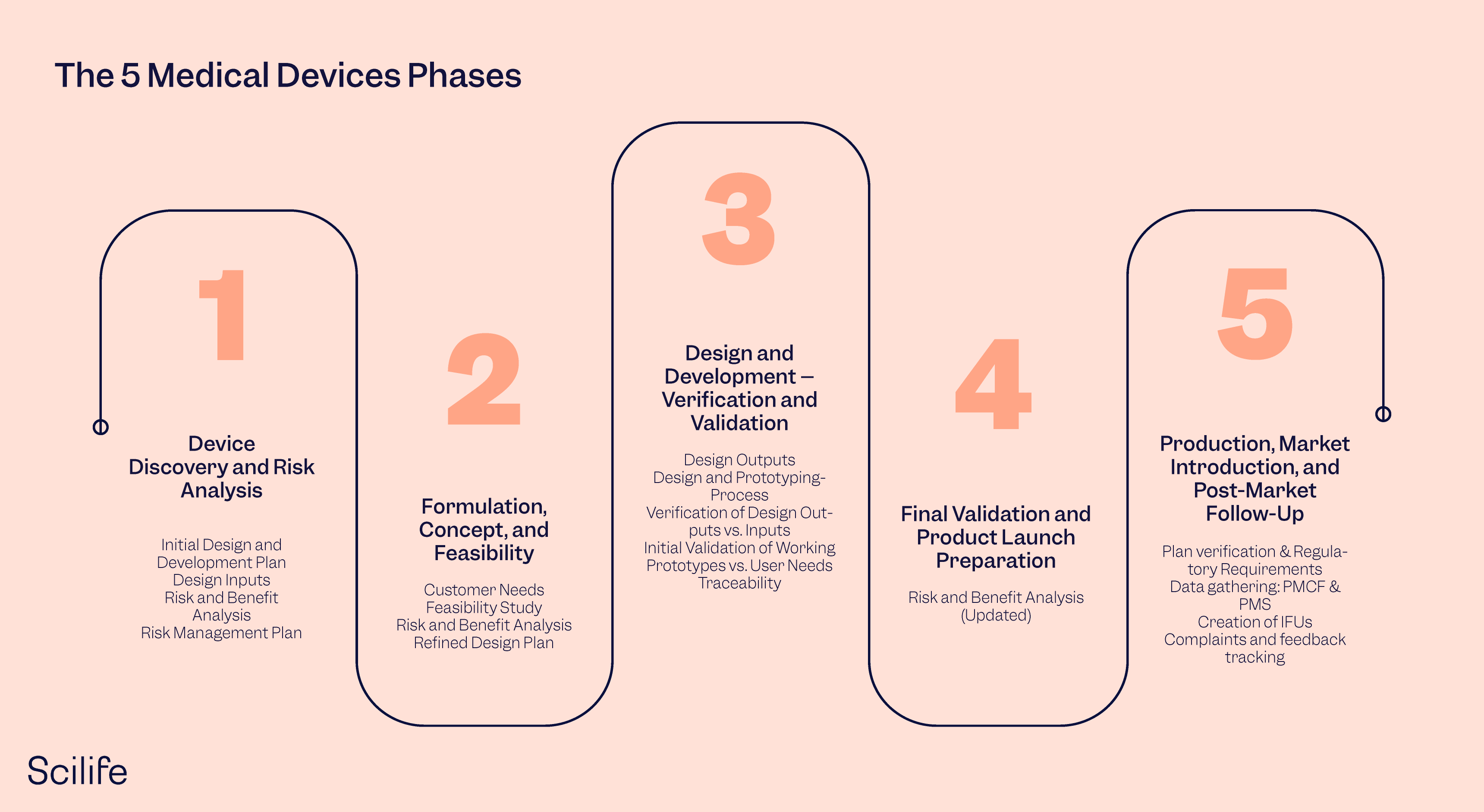 Image Source : mutors.com
Image Source : mutors.com Pin On Chethan PPT
 Image Source : www.pinterest.com
Image Source : www.pinterest.com medical device development process management lifecycle risk control ppt
10 Best 10 Medical Device Development Regulation And Law Of 2021 Of 2021
 Image Source : www.resourcecenterchicago.org
Image Source : www.resourcecenterchicago.org ISBN 9780996346276 - Medical Device Development: Regulation And Law 4th
 Image Source : www.directtextbook.com
Image Source : www.directtextbook.com Pin on chethan ppt. Sell, buy or rent medical device development: regulation and law. Medical device development: regulation and law 2020. Medical device development : regulation and law by jonathan s. kahan. Medical device development process management lifecycle risk control ppt