Ivdr Regulation Specific Details : What it is
The new IVDR (In Vitro Diagnostic Regulation) is a regulation issued by the European Union (EU) that aims to ensure the safety and reliability of in vitro diagnostic medical devices. This regulation introduces stricter requirements and regulations for manufacturers, notified bodies, and other stakeholders in the field of in vitro diagnostics. In this article, we will provide you with specific details about the IVDR and how it impacts the healthcare industry.
What is the IVDR?

The IVDR, or In Vitro Diagnostic Regulation, is a new regulation introduced by the European Union to improve the safety, performance, and reliability of in vitro diagnostic medical devices. This regulation applies to all manufacturers, importers, and distributors of in vitro diagnostic devices that are intended for sale within the European market.
Key Changes and Requirements

The new IVDR brings several key changes and requirements that manufacturers of in vitro diagnostic devices need to comply with. These changes aim to enhance the quality and safety of these devices and ensure their proper functioning. Some of the key changes and requirements introduced by the IVDR include:
- Classification System: The IVDR introduces a new risk-based classification system for in vitro diagnostic devices. This system categorizes devices into different classes based on their potential risks to patients and users.
- Increased Scrutiny of High-Risk Devices: High-risk in vitro diagnostic devices will be subject to a more rigorous assessment and scrutiny process. This includes the involvement of notified bodies, which are designated organizations responsible for assessing conformity with the IVDR requirements.
- Stricter Clinical Evaluation and Performance Studies: The IVDR imposes stricter requirements for the clinical evaluation and performance studies of in vitro diagnostic devices. Manufacturers are required to provide clinical evidence supporting the safety and effectiveness of their devices.
- Unique Device Identification (UDI) System: The IVDR introduces a UDI system, which requires unique identification of each in vitro diagnostic device. This system aims to enhance traceability and improve the monitoring of medical devices throughout their lifecycle.
- Increased Post-Market Surveillance: The IVDR mandates manufacturers to have robust post-market surveillance systems in place. This includes monitoring the performance and safety of their devices once they are in the market and ensuring timely reporting of any adverse events or non-compliance.
Frequently Asked Questions (FAQ)
1. How does the IVDR impact medical device manufacturers?
The IVDR imposes stricter requirements and regulations on medical device manufacturers. It requires manufacturers to invest more resources in clinical evaluation, performance studies, and post-market surveillance. The new classification system also means that some devices that were previously considered low-risk might now fall into higher-risk categories, requiring additional scrutiny.
2. What are the benefits of the IVDR?
The IVDR aims to enhance the safety, reliability, and effectiveness of in vitro diagnostic devices. By imposing stricter requirements and improving the assessment and monitoring processes, the IVDR ensures that patients and healthcare professionals can have confidence in the quality and performance of these devices. The UDI system also improves traceability, making it easier to identify and remove any faulty or unsafe devices from the market.
3. When does the IVDR come into effect?
The IVDR was published in May 2017 and entered into force on May 25, 2017. However, due to the complex nature of the regulation and the need for manufacturers to comply with the new requirements, the IVDR includes a transition period. The full application of the IVDR is scheduled to begin on May 26, 2022.
These are just a few of the key details and changes introduced by the IVDR. It is important for manufacturers and other stakeholders in the healthcare industry to thoroughly understand and comply with these requirements to ensure the safety and effectiveness of in vitro diagnostic devices in the European market.
For more information and detailed guidance on IVDR compliance, consult the official publications and resources provided by the European Union.
IVDR Consulting Service | EU In Vitro Diagnostic Regulation IVDR Training
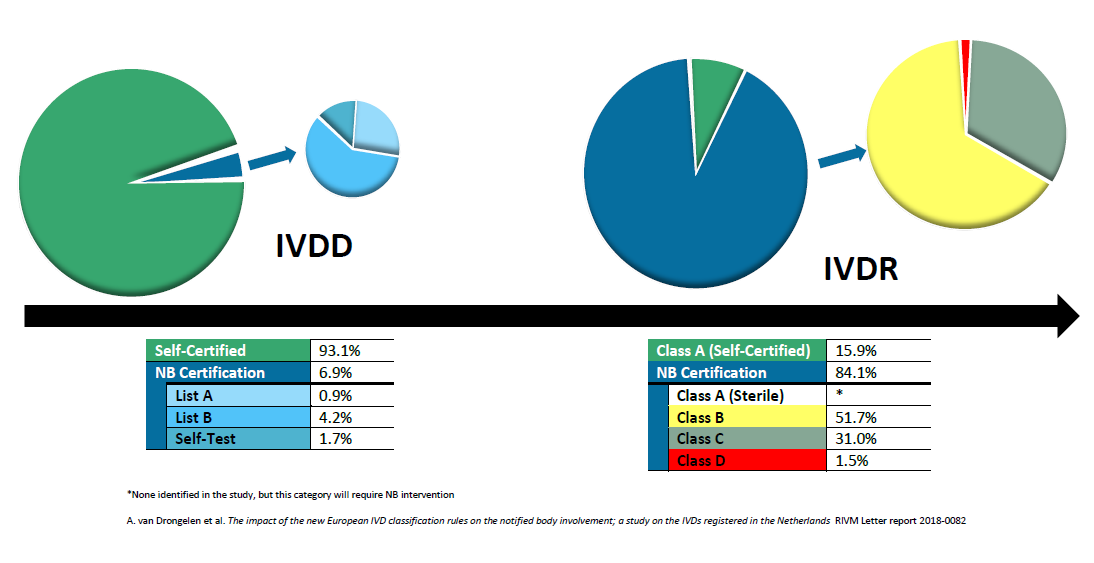 Image Source : www.fangconsulting.com
Image Source : www.fangconsulting.com ivdr vitro ivd regulation regulatory notified certification diagnostic diagnostics
In Vitro Diagnostic Regulation (IVDR) - PerkinElmer
 Image Source : rh.perkinelmer.com
Image Source : rh.perkinelmer.com What Is The IVDR?
 Image Source : www.phgfoundation.org
Image Source : www.phgfoundation.org ivdr regulation regulatory classification ivds adapted mhra
Umsetzung Der IVDR (In Vitro Diagnostic Regulation) Geplant Für 2022
 Image Source : www.diasys-diagnostics.com
Image Source : www.diasys-diagnostics.com In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746 - Omcmedical.com
 Image Source : omcmedical.com
Image Source : omcmedical.com EU IVDR Regulation, IVDR Compliance, In Vitro Diagnostic Regulation (IVDR)
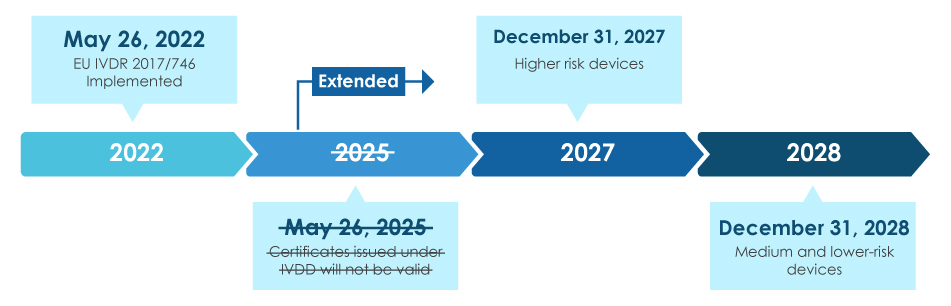 Image Source : medicaldevices.freyrsolutions.com
Image Source : medicaldevices.freyrsolutions.com ivdr regulation vitro ivds overview medicaldevices
IVDR: The EU’s In Vitro Diagnostic Regulation For Medical Diagnostic
 Image Source : www.clinicallab.com
Image Source : www.clinicallab.com (PDF) The In-vitro Diagnostics Regulation (IVDR): From Oversight To
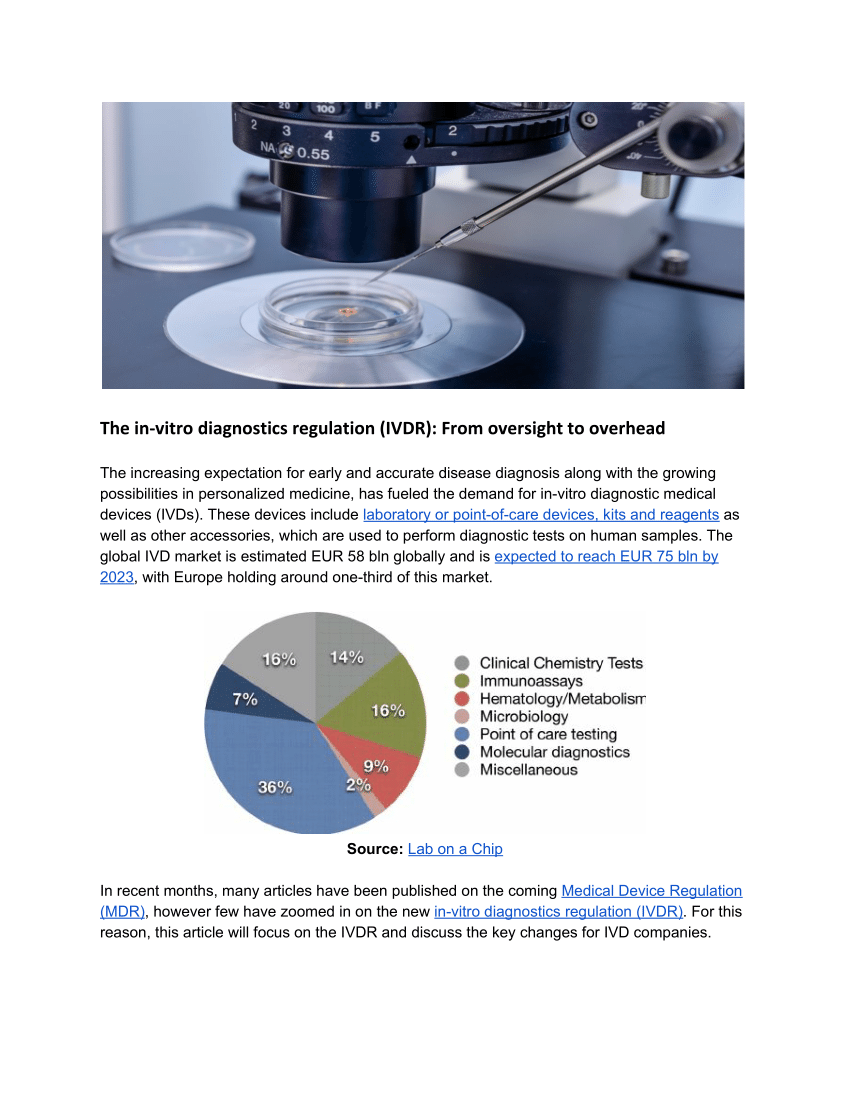 Image Source : www.researchgate.net
Image Source : www.researchgate.net ivdr regulation vitro diagnostics overhead oversight pdf
Ivdr: the eu’s in vitro diagnostic regulation for medical diagnostic. Eu ivdr regulation, ivdr compliance, in vitro diagnostic regulation (ivdr). What is the ivdr?. In vitro diagnostic regulation (ivdr). Ivdr vitro ivd regulation regulatory notified certification diagnostic diagnostics