Clinical Trial Regulation Q&A
When it comes to the Clinical Trial Regulation (CTR), there are often many questions and concerns that arise. In this post, we will address some of the most frequently asked questions regarding the CTR and provide you with the answers you need. So, let's dive right in!
1. What is the Clinical Trial Regulation?
The Clinical Trial Regulation is a regulation set forth by the European Union (EU) that aims to streamline and harmonize the rules for conducting clinical trials within the EU. It replaces the previous Clinical Trials Directive and introduces several key changes and enhancements to ensure patient safety and increase transparency in clinical trials.

Image source: TrialAssure
2. How does the Clinical Trial Regulation impact the conduct of clinical trials?
The Clinical Trial Regulation introduces several changes that impact the conduct of clinical trials. One of the key changes is the requirement for a single EU portal and database for the submission and assessment of clinical trial applications. This aims to simplify the process and enhance efficiency. Additionally, the regulation introduces new rules on informed consent, transparency, and the reporting of adverse events.
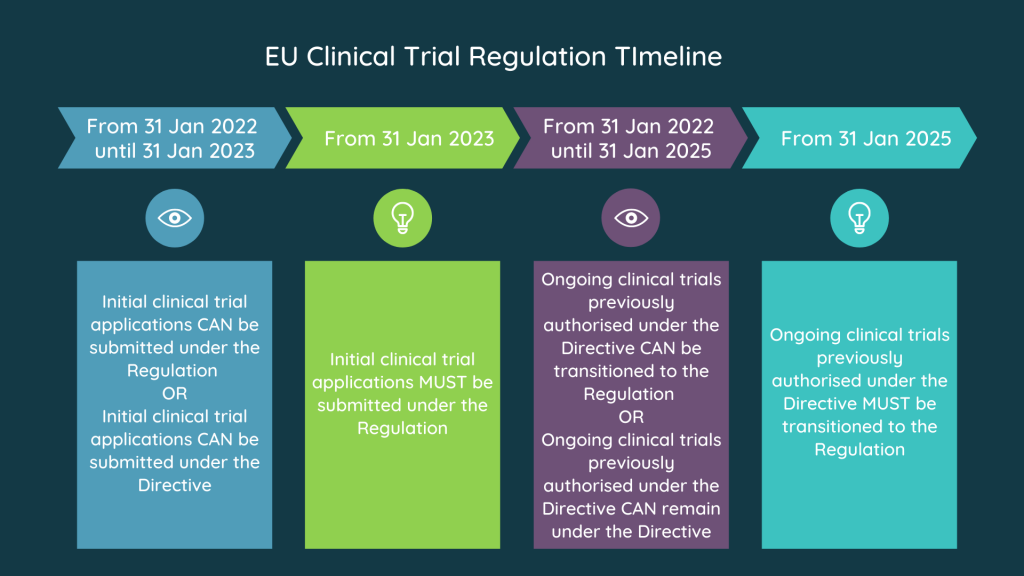
Image source: S-cubed Global
3. What are the timelines for implementing the Clinical Trial Regulation?
The implementation of the Clinical Trial Regulation has been delayed several times but is currently set to come into effect in 2022. The specific timelines may vary depending on the country and the type of clinical trial. It is important to stay up-to-date with the latest developments and ensure compliance with the new regulations.
Subheading 1: Key Changes Introduced by the Clinical Trial Regulation
Under the Clinical Trial Regulation, several key changes have been introduced to enhance patient safety, improve transparency, and streamline the conduct of clinical trials. These changes include:
- Single EU portal and database for the submission and assessment of clinical trial applications
- Stricter requirements for informed consent
- Greater transparency in the reporting of clinical trial results
- New rules on the reporting and management of adverse events
Subheading 2: Benefits of the Clinical Trial Regulation
The Clinical Trial Regulation brings several benefits to the field of clinical research. These benefits include:
- Streamlined and harmonized rules for conducting clinical trials in the EU
- Enhanced patient safety through stricter requirements for informed consent
- Improved transparency in the reporting of clinical trial results
- Increased efficiency in the submission and assessment of clinical trial applications
Subheading 3: Frequently Asked Questions
Q1: How will the introduction of a single EU portal and database impact the efficiency of clinical trial submissions?
A1: The introduction of a single EU portal and database is expected to greatly enhance the efficiency of clinical trial submissions. By centralizing the submission and assessment process, it will reduce duplication of efforts and streamline the overall process. This will save time and resources for both sponsors and regulatory authorities.
Q2: What are the main changes regarding informed consent under the Clinical Trial Regulation?
A2: The Clinical Trial Regulation introduces stricter requirements for informed consent. It emphasizes the need for clear and comprehensive information to be provided to trial participants, ensuring they understand the nature of the trial and its potential risks and benefits. Informed consent must be given freely and be based on sufficient information.
Q3: How will the Clinical Trial Regulation improve transparency in the reporting of clinical trial results?
A3: The Clinical Trial Regulation aims to improve transparency in the reporting of clinical trial results by requiring all trial results to be disclosed in the EU clinical trials database within specified timelines. This ensures that the results of clinical trials are readily accessible to healthcare professionals, researchers, and the general public, promoting accountability and preventing selective reporting of trial outcomes.
Conclusion
The Clinical Trial Regulation brings significant changes to the way clinical trials are conducted within the EU. It aims to enhance patient safety, increase transparency, and streamline the overall process. By understanding the key changes and requirements introduced by the Clinical Trial Regulation, stakeholders in the field of clinical research can ensure compliance and contribute to the advancement of medical knowledge and patient care.
We hope this post has provided you with valuable insights into the Clinical Trial Regulation. If you have any further questions or need assistance with navigating the new regulations, feel free to reach out to us. Stay tuned for more updates and information on the ever-evolving landscape of clinical trials!
The Upcoming Clinical Trial Regulation And EMA Policy 0070 Restart? Are
 Image Source : rlsciences.com
Image Source : rlsciences.com EU Clinical Trial Regulation - S-cubed Global
 Image Source : www.s-cubed-global.com
Image Source : www.s-cubed-global.com New EU Clinical Trial Regulation And What It Means For Sponsors - Life
 Image Source : www.lslaw.co.uk
Image Source : www.lslaw.co.uk New Regulation On Clinical Trials In Spain - Leon Research | CRO
 Image Source : leonresearch.com
Image Source : leonresearch.com clinical regulation cro
Clinical Trial Regulation 536 2014
 Image Source : www.slideshare.net
Image Source : www.slideshare.net Clinical Trial Regulation 536 2014
 Image Source : www.slideshare.net
Image Source : www.slideshare.net EU Clinical Trial Regulation - S-cubed Global
 Image Source : www.s-cubed-global.com
Image Source : www.s-cubed-global.com Clinical Trial Information System(CTIS) | How To Train User For New
 Image Source : www.trialassure.com
Image Source : www.trialassure.com Clinical trial information system(ctis). Eu clinical trial regulation. Clinical trial regulation 536 2014. Eu clinical trial regulation. The upcoming clinical trial regulation and ema policy 0070 restart? are