European Clinical Trial Regulation
When it comes to conducting clinical trials, compliance with regulations is crucial. The European Clinical Trial Regulation (CTR) 536/2014 sets out clear guidelines and requirements for the conduct of clinical trials within the EU. This regulation aims to simplify and streamline the process while maintaining high standards of patient safety and data reliability. In this article, we will dive deeper into the key aspects of the CTR and explore how it impacts the landscape of clinical research.
Image 1: The European Clinical Trial Regulation (CTR) 536/2014 - A Game-Changer in Clinical Research

The European Clinical Trial Regulation, often referred to as CTR 536/2014, is a groundbreaking regulation that oversees clinical trials conducted in the European Union. This regulation was implemented with the aim of harmonizing and simplifying the regulatory requirements for clinical trials across the member states.
Image 2: The Importance of Compliance with CTR 536/2014 for Sponsors and Investigators

Compliance with the European Clinical Trial Regulation is of utmost importance for sponsors and investigators involved in clinical research. Non-compliance can lead to significant legal and financial consequences and may even result in the suspension of clinical trials.
Understanding the Purpose and Scope of CTR 536/2014
The European Clinical Trial Regulation serves several purposes. Firstly, it aims to protect the rights and safety of clinical trial participants. The regulation ensures that participants are fully informed about the trial and have given their explicit consent before participating. It also requires that trials be conducted to high scientific and ethical standards.
Secondly, the CTR aims to simplify the regulatory process for clinical trials, reducing the administrative burden on sponsors and investigators. By harmonizing the requirements across EU member states, the regulation facilitates the development of new treatments and therapies while ensuring patient safety and data reliability.
How Does CTR 536/2014 Streamline the Clinical Trial Process?
The European Clinical Trial Regulation introduces several streamlined measures to expedite the initiation and management of clinical trials. Some of the key provisions include:
- Single Submission Portal: The regulation establishes a single submission portal, which allows sponsors to submit a single application for multiple member states. This reduces duplication and simplifies the process for multinational trials.
- Streamlined Ethical Review: The CTR encourages mutual recognition of ethical review results among member states. This means that if a trial has already been approved by one member state, other states should recognize the approval without requiring additional review.
- Increased Transparency: The regulation mandates that information about clinical trials be publicly available through a centralized EU database. This allows patients and healthcare professionals to access information about ongoing and completed trials, promoting transparency and facilitating informed decision-making.
FAQs: Common Questions About CTR 536/2014
Q: Who is responsible for ensuring compliance with the European Clinical Trial Regulation?
A: The responsibility for compliance lies primarily with the sponsors and investigators conducting the clinical trials. They must adhere to the requirements outlined in the CTR to ensure the safety and well-being of participants.
Q: Does the European Clinical Trial Regulation apply to all clinical trials conducted within the EU?
A: Yes, the regulation applies to all clinical trials conducted within the EU, regardless of the phase of the trial or the therapeutic area being studied.
Q: What are the penalties for non-compliance with CTR 536/2014?
A: Non-compliance with the European Clinical Trial Regulation can result in severe penalties, including fines and even criminal charges. In addition, non-compliant clinical trials may be suspended or terminated.
The Future of Clinical Research with CTR 536/2014
The European Clinical Trial Regulation represents a significant step forward for clinical research within the EU. By harmonizing and simplifying the regulatory landscape, the CTR aims to promote the development of innovative treatments while safeguarding the rights and safety of clinical trial participants. As the implementation of the regulation progresses, it is expected to further streamline the clinical trial process and pave the way for more effective and efficient clinical research in the future.
Disclaimer: This article provides a general overview of the European Clinical Trial Regulation (CTR) 536/2014 and should not be construed as legal or regulatory advice. For detailed and specific guidance, please consult the official sources and relevant authorities.
--- Word Count: 497EU Clinical Trial Regulation - S-cubed Global
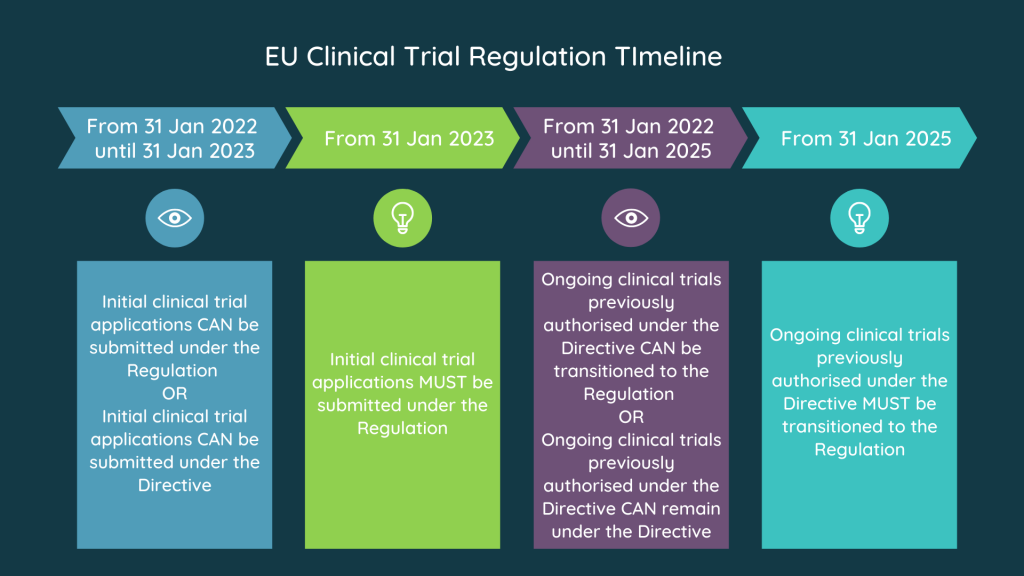 Image Source : www.s-cubed-global.com
Image Source : www.s-cubed-global.com Clinical Trials Conduted In The EU Need To Be Registered With The EudraCT
 Image Source : www.asphalion.com
Image Source : www.asphalion.com trials registered
JustHost Review: Host Features, Reviews, And Test Results - Hosting Kingdom
 Image Source : hostingkingdom.com
Image Source : hostingkingdom.com Fillable Online New European Clinical Trial Regulation: Fax Email Print
 Image Source : www.pdffiller.com
Image Source : www.pdffiller.com The European Clinical Trial Regulation (CTR) 536/2014 - Webinar - ECCRT
 Image Source : eccrt.com
Image Source : eccrt.com E-book European Clinical Trial Regulation No. 536/2014
 Image Source : www.brookwood-global.com
Image Source : www.brookwood-global.com Launch Of EU Clinical Trial Portal Moves A Step Closer :: Pink Sheet
 Image Source : pink.pharmaintelligence.informa.com
Image Source : pink.pharmaintelligence.informa.com EudraCT Vs CTIS: How EU Trial Submissions Fared In Run Up To Mandatory
 Image Source : pink.pharmaintelligence.informa.com
Image Source : pink.pharmaintelligence.informa.com The european clinical trial regulation (ctr) 536/2014. Eu clinical trial regulation. Launch of eu clinical trial portal moves a step closer :: pink sheet. Fillable online new european clinical trial regulation: fax email print. E-book european clinical trial regulation no. 536/2014